*Sponsored
Krypton Street Initiates Coverage On (OKYO) Starting This Morning
—Friday, August 1, 2025
(OKYO) Comes Backed By Several Potential Catalysts Including:
Three Analysts Now Cover (OKYO) With Targets Ranging From $7 To $13, With The Most Recent Suggesting Over 420% Upside Potential
(OKYO) Moved Approximately 245% Inside of 6 Months—From
Late January To Mid-June
Public Float Under 25.1 Mln Shares May Amplify The Potential For Big Moves If Demand Begins To Shift
11 Bullish Indicators Lit Up On Barchart Across Short-, Medium-, And Long-Term Timeframes As Of Yesterday
Take A Look At (OKYO) This Morning While It's Still Early…
August 1, 2025
Early Coverage | See Why (OKYO) Just Hit Our Radar This Moring Dear Reader, Something rare is unfolding behind the scenes—an under-the-radar biotech is stepping into a space where no approved treatment exists… and the clock is already ticking. With major analysts weighing in and fast-track status already secured, momentum may be building ahead of a pivotal moment. While the race in pharma often favors the loudest or largest, OKYO Pharma (NASDAQ: OKYO) is quietly targeting a pain disorder with no FDA-approved treatment—and they may be first to the gate. Right now, this little-known biopharma is advancing a compound known as Urcosimod, formerly OK-101, that could redefine the treatment approach for a devastating and largely overlooked condition: neuropathic corneal pain (NCP). It's not just the science drawing attention—multiple analysts are stepping in with targets that reflect what may be shaping up behind the curtain. And if even one of these projections proves accurate, what's coming next could shift this name from overlooked to in demand—fast. Recent Analyst Target Suggests Over 420% Upside Potential
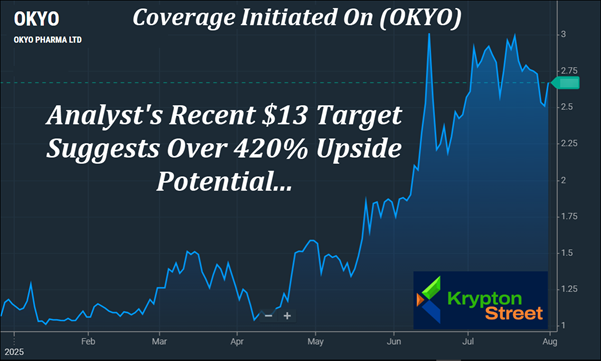
Just yesterday, on July 31, Elemer Piros, PhD, from Lucid Capital Markets, initiated coverage on OKYO with a $13 target—a figure that implies over 420% upside potential from its recent levels. That follows Goldman Small Cap Research's updated report on June 11, which raised its 6–12 month target to $8, which suggests over 220% upside potential. And earlier this year, Yi Chen, Ph.D., CFA, at H.C. Wainwright & Co., set a 12-month target of $7, which suggests over 180% upside potential from recent levels. Beyond the analyst confidence, there are clear structural dynamics at play: - Yahoo Finance reports less than 25.1 Mn shares in the public float—a key metric for volatility potential.
- Over the past 6 months, (OKYO) moved approximately 245%, from $0.90 on January 30 to $3.11 on June 16.
- As of July 31, Barchart's technical opinion tool was flashing 11 Bullish Signals across short-, mid-, and long-term timeframes.
Put simply—between high-profile analyst coverage, a low float, and a technical setup already lighting up the radar, (OKYO) is entering the next phase of its clinical campaign with serious momentum. Why NCP Matters—And Why (OKYO) May Be on the Brink of a Breakthrough

NCP isn't your average eye discomfort. It's a chronic, debilitating condition where patients experience severe ocular pain in the absence of visible damage—often after failed interventions for dry eye disease (DED). These individuals live in a limbo between normal diagnostics and relentless pain. (OKYO)'s Urcosimod—a novel lipid-conjugated peptide targeting the ChemR23 receptor—has shown compelling activity in preclinical and early human data. This candidate appears to modulate both inflammatory and neuropathic pain pathways, offering dual-action potential in a field that's been starved for innovation. Here's where it gets more interesting: - In January 2024, Urcosimod completed a 240-patient DED trial, showing strong signs of ocular pain relief.
- In October 2024, (OKYO) initiated a first-of-its-kind Phase 2 trial for NCP with FDA clearance in hand.
- By April 2025, with 17 patients having completed the study, OKYO announced an early closure of the trial—triggered by the company's eagerness to access the masked data.
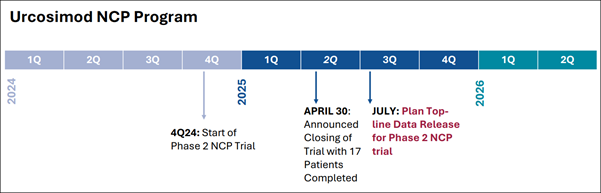
This wasn't a failure—it was a calculated move to accelerate timelines and potentially fast-track the review process.
Just a day later, the FDA granted Fast Track Designation to Urcosimod for NCP—a rare nod of urgency and support for a therapy with strong potential to meet an unmet need. The Science That Sets It Apart
What makes Urcosimod stand out isn't just what it targets—but how it works. This next-gen compound is based on a natural molecule the body uses to manage inflammation. Scientists modified it to home in on specific pain and immune receptors found in both the eye and the nervous system—giving it the potential to tackle inflammation and nerve pain at the same time. Here's what else makes it different: - It's a once-a-day eye drop—no needles, no pills, just a simple topical dose.
- It uses a special sticky formula to stay on the eye longer—so more of the treatment is absorbed where it matters.
- In early tests, it showed pain relief similar to major prescription meds like gabapentin—but without the systemic side effects.
For doctors and patients alike, this could be a major shift—a targeted eye drop for a condition that's never had a real solution. What Just Happened in the Trial
The latest study on this potential NCP breakthrough was run by a leading eye pain expert at Tufts Medical Center. Participants were split into three groups—some received low or high doses of the compound, while others received a placebo. Treatment lasted 12 weeks, with pain tracked closely throughout the process. The trial was shut down early—not due to issues, but to speed up review of the results. And now, the results are in…
OKYO Pharma Reports Strong Results for Its Lead Eye Pain Treatment
(OKYO) just shared positive news: its experimental eye drop, Urcosimod, showed powerful results in reducing pain for patients suffering from neuropathic corneal pain (NCP)—a severe eye condition with no FDA-approved treatments available today. In the recent clinical trial: - 75% of patients who received the lower-dose version of the eye drop saw their pain drop by over 80% after just 12 weeks.
- Pain relief started showing up as early as Week 4 and stayed strong through the end of the trial.
- The patients in this trial had already tried other treatments without success, making these results especially promising.
- No serious side effects were reported in any of the patients.
The company now plans to meet with the FDA to map out next steps. Since Urcosimod already has Fast Track status, the approval process could move faster than usual. 
The trial, conducted at Tufts Medical Center, was led by a top expert in eye pain and ended early—not due to problems, but because the early results were so promising that (OKYO) wanted to begin the next phase sooner. Several patients from the trial have already requested continued access to Urcosimod through the FDA's Compassionate Use program—another sign of real impact. (OKYO) is aiming to be the first to bring a treatment for this painful condition to market. With growing attention from patients and analysts alike, the company says it's now working quickly to move forward with further development. Momentum is building—and the setup unfolding here isn't just clinical, it's structural. With pressure mounting from multiple angles, this one is flashing all the signs of a potential breakout in the making. 
7 Reasons Why OKYO Pharma (NASDAQ: OKYO) Is Topping Our Watchlist
This Morning—Friday, August 1, 2025
1. Multi-Analyst Coverage: (OKYO) is now covered by three independent analysts, with targets ranging from $7 to $13, with the highest suggesting over 420% upside potential from its recent range.
2. Recent Momentum: Over the last 6 months, (OKYO) moved approximately 245%—from $0.90 on January 30 to $3.11 on June 16—reflecting increased interest around upcoming clinical readouts. 3. Low Float Setup: With fewer than 25.1 Mln shares in the public float, (OKYO) has the potential to witness big swings if demand begins to shift. 4. Rare First-Mover: (OKYO) is targeting a severe ocular condition—neuropathic corneal pain—that currently has no FDA-approved treatment. 5. Fast Track Approval: The FDA has granted Fast Track status to (OKYO)'s lead program, a recognition typically reserved for candidates addressing critical unmet needs. 6. Trial Acceleration + FDA Engagement: After dosing just 17 patients in its Phase 2 trial, (OKYO) closed enrollment early to access data sooner—signaling urgency and confidence. Topline results released in July showed compelling efficacy, and the company now plans to meet with the FDA to discuss potential next steps under its Fast Track designation. 7. Technical Bullishness: As of yesterday, July 31, (OKYO) was triggering 11 bullish signals on Barchart, spanning short-, medium-, and long-term indicators. Momentum may already be shifting—and if the FDA discussion leads to even a hint of regulatory clarity, this could trigger the next leg up. We've seen setups like this before… and when all the pieces line up, the move doesn't wait around. Take A Look At (OKYO) While It's Still Early…
With a rare disease focus, fast-track clearance, and analysts now stepping in with targets as high as $13, OKYO Pharma (NASDAQ: OKYO) could start to attract serious attention. Between its small float, accelerating clinical path, and early technical strength, this one checks multiple boxes for those tracking potential near-term biotech catalysts. This just landed on our radar—and with topline results now public, all eyes are turning to what happens next with the FDA. We have all eyes on (OKYO) this morning. Take a look at (OKYO) while it's still early. Keep an eye out for my next update—it could be hitting within the next 60-90 minutes.
Sincerely,
Alex Ramsay
Co-Founder / Managing Editor
Krypton Street Newsletter | 






No comments:
Post a Comment