*Sponsored
Market Crux Announces Coverage On Tiziana Life Sciences (Nasdaq: TLSA) Starting Right Now—Wednesday, July 30, 2025
(TLSA) Comes Backed By Several Potential Catalysts Like:
Lucid Capital Recently Initiated Coverage On (TLSA) With A Target Of $8, Which Suggests Over 300% Upside Potential.
(TLSA) Went From $0.63 To $1.98 Inside Of Just Six Months, A Move Of Approximately 214%.
The Executive Chairman Increased His Stake To Over 43 Mln Shares,
Exceeding 37% Ownership.
(TLSA) Is Now Trending Above Its 5-, 20-, 50-, 100-, And 200-Day
Moving Averages.
Take A Look At (TLSA) This Morning—While It's Still Quiet…
July 30, 2025 Chart Watch | (TLSA) Jumps To The Top Of Our Early Watchlist—Here's Why Dear Reader, Are you watching (TLSA) right now? (TLSA) just tapped $2.15 on an approximate 9% early move—marking a new 52-week high according to Barchart. 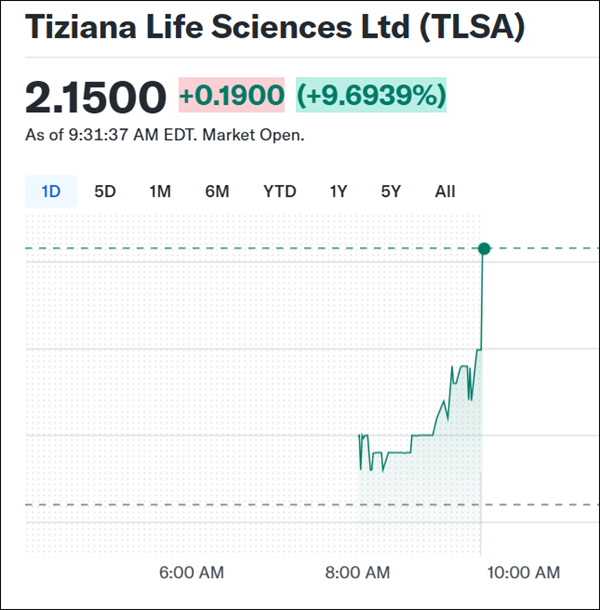
But that's not all, (TLSA) is now lighting up 100% Bullish Signals on Barchart's Technical Opinion Tool, across its TrendSeeker, Short, Medium, and Long-Term Indicators Did you miss my email from earlier? If you did, now's the time to quickly get up to speed—keep reading to see why we're so excited to be bringing this to you right now. Some setups don't just stand out—they break the mold. They aren't following a script or recycling what's already been done. They're building something new—something with the potential to reset expectations across an entire category. That's exactly what we're seeing with Tiziana Life Sciences (Nasdaq: TLSA). While others in the biotech space continue chasing familiar paths, (TLSA) is rewriting the playbook—developing a novel approach to immunotherapy that targets the brain and central nervous system with precision, without triggering full-body immune disruption. This isn't just theory. It's backed by emerging clinical evidence, growing analyst recognition, and a sharp technical setup. Over the past six months, (TLSA) has moved approximately 214%—from $0.63 on February 6 to $1.98 on July 29—and now trends above all five of its major moving averages, including the 5, 20, 50, 100, and 200-day indicators tracked by Barchart. Something real is taking shape here—and the timing may matter more than most realize. Analyst Initiates Coverage: Target Suggests Over 300% Upside Potential 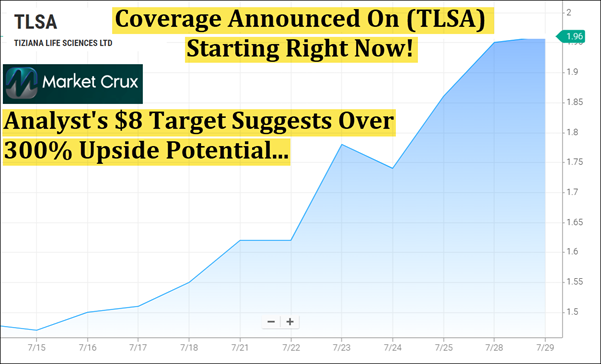
Last week, Lucid Capital Markets initiated coverage on Tiziana Life Sciences (TLSA) with a bullish rating and an $8 target—which suggests over 300% upside potential from recent levels. The core of their thesis centers on foralumab's potential to do more than manage symptoms; it may actually modify disease activity. No FDA-approved treatments currently address non-active SPMS directly, making this a rare opening. Lucid estimates U.S. net sales could reach $1.6B, with EU5 projections approaching $1B by 2030—assuming 50% market share. They also note that Tiziana's commercial window could extend into the 2040s, giving this program time to scale.
Lucid draws a bold comparison: positioning (TLSA)'s foralumab as a potential "Humira for CNS disorders." That's not casual language—Humira became the world's best-selling therapy for years, generating over $20B annually at its peak. The Core Technology: Nasal Foralumab, the First of Its Kind
At the heart of (TLSA)'s program is foralumab, a fully human anti-CD3 monoclonal antibody. But this isn't your typical mAb. Instead of the standard IV infusion that comes with systemic immune risks and long clinic visits, (TLSA) is pioneering a nasal delivery system. It's non-invasive. It bypasses the bloodstream. And it targets the mucosal immune system directly—where it can activate regulatory T cells and tamp down inflammation without triggering a full-body immune response. This approach could represent a leap forward in immunomodulation. And it's already showing signs of real-world benefit. What's Happening in Alzheimer's: Inflammation Down, Engagement Up
In July 2025, (TLSA) dropped major news: in a patient with moderate Alzheimer's disease, nasal foralumab not only showed safety and tolerability—it was linked with measurable immune changes and visible reductions in brain inflammation. 
PET scans demonstrated decreased microglial activation—an essential marker for neuroinflammation. Even more intriguing? An increase in phagocytosis markers in classical monocytes—suggesting foralumab could assist the body's own immune cells in clearing amyloid plaques, a hallmark of Alzheimer's pathology. The patient's condition didn't just improve biologically. According to the family, behavior and engagement improved, too. And the patient has since opted to continue therapy. A Strategic Expansion into Multiple Sclerosis (MS)
Alzheimer's isn't the only space where foralumab is being deployed. (TLSA) is currently advancing a Phase 2 randomized, double-blind, placebo-controlled trial in non-active Secondary Progressive Multiple Sclerosis (na-SPMS)—an area where current therapies fall short. The trial is underway across top-tier research centers, including Brigham and Women's, Yale, Johns Hopkins, and most recently, Weill Cornell. In a prior 10-patient observational study, the results were eye-catching: - Disease stability or improvement in all patients over 6 months
- Imaging showed reduced microglial inflammation
- Fatigue reduction in 6 of 10 patients
- No new lesions on MRI
Most importantly, no serious adverse effects were reported. Why This Could Matter
Right now, patients with non-active SPMS have few viable options. Alzheimer's therapies continue to fall short, particularly in moderate-stage disease. And ALS continues to challenge researchers worldwide. (TLSA)'s foralumab—delivered intranasally—might be the outlier that breaks the cycle. It's already showing immune modulation, CNS penetration, safety, and early efficacy signals across multiple conditions. More than that, it's doing so with a patented delivery method, a clean safety profile, and the potential for broad pipeline expansion into other neuroinflammatory diseases. One More Thing: Insider Confidence In May 2025, Tiziana's Executive Chairman Gabriele Cerrone acquired 15K more shares on the open market, bringing his total stake to over 43 Mln shares, or more than 37% of the company's issued capital. That kind of long-term skin in the game isn't something you see every day. It's not just "the science." It's the alignment—on the chart, in the clinic, and inside the boardroom. From insider conviction to technical strength and expanding third-party validation, there could be a pattern forming here. And if you're tracking where attention could shift next, this setup deserves to be front and center. 7 Reasons Why (TLSA) Just Hit Our Radar This Morning—Wednesday,
July 30, 2025…
1. Analyst Coverage: Last week, Lucid Capital Markets initiated coverage on (TLSA) with a target of $8—projecting over 300% upside from recent levels.
2. Recent Momentum: (TLSA) went from $0.63 to $1.98 over the past 6 months—an approximate 214% move, reflecting sustained momentum on the chart. 3. Technical Signals: (TLSA) is trending above its 5, 20, 50, 100, and 200-day moving averages, confirming strong short- and long-term alignment. 4. Insider Confidence Deepens: In May, (TLSA)'s Executive Chairman increased his position to over 43 Mln shares—representing more than 37% of issued capital. 5. Real Impact in Alzheimer's: PET imaging from (TLSA)'s intranasal foralumab program showed reduced brain inflammation and immune modulation in a treated patient. 6. Immune-Targeting Innovation: (TLSA)'s nasal delivery avoids systemic immune disruption and may offer a safer path to CNS immune regulation across multiple diseases. 7. Clinical Sites Keep Growing: (TLSA)'s Phase 2 trial in non-active SPMS is now live at top-tier centers including Weill Cornell, Yale, and Johns Hopkins. The potential for momentum could pick up. Names like this don't stay quiet for long—especially when the setup checks this many boxes. The timing here couldn't be more critical. Take A Look At (TLSA) This Morning—While It's Still Quiet…
Tiziana Life Sciences (Nasdaq: TLSA) isn't another biotech trying to push recycled therapies into crowded indications. It's doing something genuinely different—and based on emerging clinical data and third-party validation, it might be on the verge of making a mark in some of the most challenging conditions in neurology. With strong chart momentum, expanding analyst coverage, insider alignment, and emerging clinical signals in areas like Alzheimer's and SPMS, Tiziana Life Sciences (Nasdaq: TLSA) is beginning to separate itself from the noise. The combination of technical strength and early scientific traction makes this a setup worth watching closely. (TLSA) just tapped $2.15 on an approximate 9% early move—marking a new 52-week high according to Barchart. Keep an eye out for my next update—it could be out any moment. Sincerely,
Gary Silver
Managing Editor,
Market Crux | 






No comments:
Post a Comment