*Sponsored
Krypton Street Announces (MBRX) As Its Next Potential Breakout Idea—Starting This Morning—Wednesday, August 27, 2025
(MBRX) Comes Backed By Several Potential Catalysts That Just Pushed It To The
Top Of Our Watchlist—But Here's What We Can Tell You Right Now…
Recent Momentum: (MBRX)'s Chart Showcases An Approximate 192% Move In Under Two Months.
Analyst Targets: Roth MKM Set A $13 Target On (MBRX), Which Suggests An
Upside Potential Of Over 2,100% From Recent Range.
Market Growth: (MBRX) Is Aligned With Oncology Markets Projected To Expand From $250B In 2025 To Nearly $668B By 2034.
Pull Up (MBRX) While It's Still Early…
August 27, 2025
Morning Watchlist | (NASDAQ: MBRX) Hits Our Radar With 7 Potential Catalysts Dear Reader, Momentum in the late-stage pharma space doesn't come often—but when it does, it tends to build fast. Right now, one name is stepping forward with global trials, breakthrough preclinical findings, and a lead therapy that's already turning heads among top researchers. The pieces are locking into place, and the setup ahead could prove to be one of the most important stories we've tracked this year. Adding weight to this, analysts have started to highlight Moleculin Biotech, Inc. (NASDAQ: MBRX)—with coverage pointing to upside potential ranging from 600% to over 2,100% based on its recent $0.57 range. 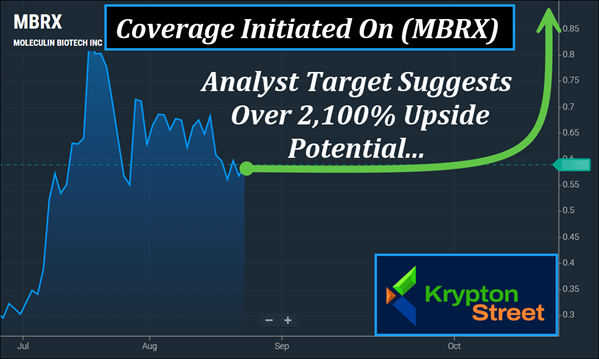
(MBRX) has already demonstrated its potential for momentum, moving approximately 192% from $0.25 on June 20 to $0.73 on August 6. Jason McCarthy at Maxim Group and Sara Nik at H.C. Wainwright each set targets of $4, while Jonathan Aschoff at Roth MKM set a $13 target. It's not hard to see why. The global oncology market—estimated around $250B in 2025—is projected to grow to nearly $668B by 2034. In the U.S. alone, oncology is set to climb from $90B in 2025 to over $220B by 2034. Against this backdrop, the AML market is expected to expand from $1.74B in 2025 to nearly $2.92B by 2032, while the CAR T-cell therapy segment could grow from $12.9B in 2025 to approximately $128B by 2034. With these markets accelerating, (MBRX) has aligned its pipeline toward some of the most urgent and underserved areas. (MBRX) has been steadily advancing through the clinic, and 2025 is shaping up as a critical year. With multiple late-stage trials underway, the company is pursuing therapies designed for some of the toughest cancers and vi-rus-related illnesses—areas where conventional approaches often fall short. At the center of this progress is Annamycin, a next-generation therapy designed to do what older chemotherapy treatments often cannot: attack cancer effectively without the heart-damaging side effects that limit traditional anthracyclines. Why Annamycin Stands Out
Annamycin—recently given the non-proprietary name naxtarubicin—was engineered to overcome two of the biggest barriers in cancer treatment: - Resistance: Over time, many cancers adapt to therapy, reducing effectiveness. Annamycin was designed to bypass these resistance pathways.
- Heart Safety: Current anthracyclines carry a dose-related risk of heart failure, forcing strict treatment limits. In trials, Annamycin has shown no evidence of this toxicity—even in patients who received multiple cycles at levels above the FDA's lifetime limit.
This combination—strong anti-cancer activity paired with a safety profile that may support repeated use—sets Annamycin apart from conventional therapies. The Lead Program: The MIRACLE Trial
The most closely watched program in Moleculin's pipeline is the MIRACLE trial, a global Phase 3 study evaluating Annamycin in combination with cytarabine—together known as AnnAraC—for patients with relapsed or refractory acute myeloid leukemia (AML). AML is an aggressive cancer, and once patients relapse, current care options remain extremely limited. That is why the progress of this trial carries such weight. Earlier studies combining Annamycin with cytarabine produced remission rates that went beyond expectations, even in patients who had already cycled through multiple lines of therapy. Building on that foundation, the FDA has provided direct guidance on the design of the MIRACLE study. The first readout of patient data is expected before the end of 2025, with additional updates anticipated in the first half of 2026. This pivotal trial could help reshape expectations for AML treatment if results continue to hold. Beyond AML: Expanding the Reach of Annamycin
Annamycin's potential reach goes well beyond AML: - Soft Tissue Sarcoma (STS) Lung Metastases: In a Phase 1B/2 study, patients who had already failed multiple prior treatments lived a median of 13.5 months after receiving Annamycin. That outcome rivaled or exceeded results typically seen in patients treated much earlier in their care cycle.
- Liver Cancers: Preclinical data presented in August 2025 showed powerful activity against primary liver cancer, colorectal cancer that spread to the liver, and pancreatic cancer with liver involvement. Importantly, Annamycin concentrated in the liver, lungs, spleen, and pancreas—suggesting a natural targeting effect.
Together, these findings highlight a broader therapeutic reach across multiple cancer types. A Pipeline Beyond Annamycin
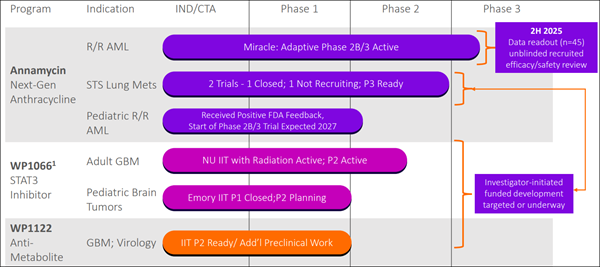
(MBRX) is advancing more than a single therapy. Its pipeline includes: - WP1066: An immune/transcription modulator designed to shut down cancer-driving proteins like p-STAT3 while boosting the body's natural immune response. Trials are underway in glioblastoma and pediatric brain tumors.
- WP1122: A compound designed to block cancer and vi-ruses from accessing the glucose they need to survive. Preclinical work is being led at Emory University.
Many of these programs are supported by investigator-led studies, allowing (MBRX) to broaden its therapeutic pipeline while conserving resources. Recent Momentum: 2025 Highlights
The past year has brought several key developments: - Global Expansion: The MIRACLE trial has now expanded into the U.S., Europe, Georgia, and Ukraine, with more than 20 additional sites expected by the end of Q3 2025.
- Patent Strengthening: Multiple new patents have extended protection for Annamycin into 2040.
- Preclinical Breakthroughs: Liver cancer data presented at MD Anderson further validated Annamycin's unique profile.
- Ca-sh Position: As of June 30, 2025, (MBRX) held $7.6M in ca-sh, supporting operations into the fourth quarter while continuing to pursue strategic partnerships and externally funded programs.
With so much lining up at once, (MBRX) is no longer just another late-stage pharma quietly moving through trials—it's quickly becoming a name that could command far more attention. The combination of recent moves, analyst coverage, and expanding clinical milestones is creating the type of setup that demands a closer look. 7 Reasons Why (MBRX) Is Topping Our Watchlist This Morning
—Wednesday, August 27, 2025
1. Recent Momentum: (MBRX) recently moved approximately 192% from $0.25 on June 20 to $0.73 on August 6, highlighting its potential for momentum. 2. Analyst Targets: Coverage on (MBRX) includes $4 targets from Maxim Group and H.C. Wainwright, and a $13 target from Roth MKM—which suggests 600% to over 2,100% upside potential. 3. Market Growth: (MBRX) is aligned with oncology markets projected to expand from roughly $250B in 2025 to nearly $668B by 2034, with AML and CAR T-cell segments also showing sharp growth. 4. Global Trials: The Phase 3 MIRACLE study puts (MBRX) on an international stage with enrollment across the U.S., Europe, Georgia, and Ukraine. 5. FDA In Focus: In studies, Annamycin from (MBRX) has shown no evidence of heart damage even at levels above the FDA's lifetime limit for anthracyclines. 6. Pipeline Breadth: Beyond Annamycin, (MBRX) is advancing additional compounds such as WP1066 for brain tumors and WP1122 for oncology and vi-ral indications. 7. Patent Strength: With new protections for Annamycin extended into 2040, (MBRX) has strengthened its intellectual property foundation for long-term development. Momentum potential like this doesn't stay under the radar for long, and (MBRX) is now stepping into a spotlight that continues to grow brighter by the day. The alignment of market growth, analyst coverage, and pivotal trial progress makes this a setup worth paying very close attention to. Pull Up (MBRX) While It's Still Early…

The story around Moleculin Biotech, Inc. (NASDAQ: MBRX) is hard to ignore right now. In just weeks, it moved approximately 192% off its June levels, underscoring the kind of momentum that gets noticed. Analysts are already weighing in with targets that stretch from $4 to $13, which suggests 600% to 2,100% upside potential from its recent range. Layer on top of that, the sheer scale of the oncology markets—projected to more than double over the next decade—and it's clear that (MBRX) is advancing within one of the fastest-growing areas in healthcare. Its Phase 3 MIRACLE trial is already enrolling patients across multiple countries, its lead therapy has shown no evidence of heart toxicity even beyond standard limits, and additional pipeline compounds are broadening its reach well past a single indication. With patents for Annamycin now extended into 2040, the foundation for long-term development continues to solidify. We have all eyes on (MBRX) this morning. Take a look at (MBRX) while it's still early. Also—my next update could be here within the next 30-45 minutes, make sure you're watching out for it. Sincerely, Alex Ramsay
Co-Founder / Managing Editor
Krypton Street Newsletter | 




No comments:
Post a Comment