*Sponsored
Krypton Street Initiates Coverage On GeoVax Labs (Nasdaq: GOVX) Starting Tomorrow Morning—Wednesday, July 30, 2025
(GOVX) Comes Backed By Several Potential Catalysts Including:
Targeted Market Segments: Crystal Research Associates Cited (GOVX)'s Programs As Addressing Markets Estimated At $30B, $15B, And $10B+.
Limited Float: (GOVX) Has Less Than 14M Shares Listed In Its Public Float Which Could Lead To The Potential For Significant Swings If Demand Begins To Shift.
Analyst Coverage: Noble Capital Markets Reaffirmed Coverage On (GOVX) With A $10 Target That Suggests Over 1,100% Upside Potential From Today's Open.
Start Your Own Research On (GOVX) Before Tomorrow Morning…
July 29, 2025 Wednesday's Watchlist | See Why (GOVX) Just Hit Our Radar For Tomorrow Morning Dear Reader, After today's profile reached $2.96, marking an approximate 30% move in less than 24 hours, we're now turning our attention to something a little more time-sensitive. The World Health Organization recently sounded the alarm again—a fast-moving strain previously contained to Central Africa is now appearing across Europe, Asia, and the U.S., from California to New Hampshire. Detection in wastewater across multiple states suggests it's spreading faster than confirmed case counts can track. Meanwhile, pressure is mounting on global health systems still relying on a single overseas source for critical biologics. That's where GeoVax Labs (Nasdaq: GOVX) comes in—with a U.S.-based platform now being positioned as a rapid-response alternative, designed for scalability and domestic deployment. This morning, (GOVX) announced an urgent acceleration of its GEO-MVA development timeline—driven by a renewed WHO emergency declaration, a record-setting surge in Clade I cases across Africa, and fresh regulatory momentum from Europe. Late last night, the company also released its Q2 2025 update—and it's packed with momentum across immunotherapy, oncology, and global health. The company recently completed clinical-grade production and was selected for potential federal support to advance its cell-line system. With fewer than 14M shares listed in the float, the stage could be set for significant swing potential if demand begins to shift. Noble Capital Markets recently set an Outperform Rating and a $10 target on (GOVX), which would suggest over 1,100% upside potential from today's (Tuesday 7/29) open. 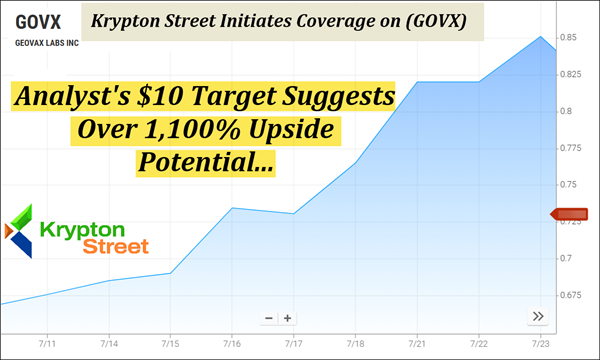
This is exactly the kind of setup that gets early attention—low float, real potential catalysts, and a spotlight that's only growing. What was once a quietly advancing platform is now being talked about in national response circles. And with federal interest building behind the scenes, the breakout may only just be getting started. Why Now? Let's Talk About the Data
• Dual-Antigen Platform in At-Risk Populations
The company's top program is being evaluated in individuals with compromised immune response—specifically patients with chronic lymphocytic leukemia (CLL). In a recent trial, (GOVX)'s approach met its primary benchmark, while a commonly used mRNA-based alternative did not. As a result, the safety board recommended continuation of the GOVX cohort and termination of the comparator arm. • Manufacturing Advantage with Platform Scalability
(GOVX)'s proprietary process uses a next-generation cell line for manufacturing—intended to be faster, more scalable, and less reliant on older, more limited production systems. Several agencies have been in discussion with the company regarding its domestic readiness and expansion potential. • Gene-Based Program for Solid Tumors (Gedeptin®)
Gedeptin® has already secured orphan designation for specific indications and is heading into a Phase 2 study paired with an immune checkpoint inhibitor. The combination is designed to target difficult-to-treat tumors, with potential application across other accessible types. GeoVax has confirmed planning is active and partnerships with academic research centers are underway. 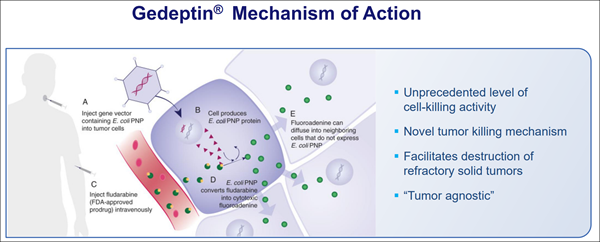
GeoVax Advances Gedeptin Combo Trial, Riding Wave Of KEYNOTE-689 Results
GeoVax Labs is refocusing its Gedeptin® clinical program to evaluate the therapy as a first-line, neoadjuvant treatment combined with pembrolizumab for primary, resectable head and neck cancer, following impressive results from the KEYNOTE-689 trial.
KEYNOTE-689's findings established perioperative pembrolizumab as a new standard, significantly boosting event-free survival rates for these patients. GeoVax aims to begin a new Phase 2 trial combining Gedeptin®, fludarabine, and pembrolizumab, targeting enhanced immune activation, better tumor clearance, and improved outcomes. This strategic move aligns Gedeptin's immune-sensitizing effects with cutting-edge immunotherapy, offering hope for high-risk and hard-to-treat cases.
GeoVax Labs: Crystal Research Report Highlights Significant Market
Scale Across Key Programs – See Full Report Here
GEO-CM04S1: Supported by a recent peer-reviewed publication, this next-gen immune platform is designed for broader and longer-lasting protection. Estimated global market: $30B+. Gedeptin®: Advancing toward Phase 2 in head and neck cases, with potential expansion into other solid tumors. Global potential for head and neck applications alone: $15B+. GEO-MVA: Clinical evaluation targeted for late 2025, offering a U.S.-based solution to international supply challenges. Estimated global market: $10B+.
Recent Developments: Global Signals, U.S. Response
On June 11, (GOVX) responded to the World Health Organization's latest global alert regarding a fast-moving Clade 1 strain that is now spreading beyond Central Africa into Europe, Asia, and multiple U.S. states. Detection of Clade 1 material in U.S. wastewater adds urgency to the concern of silent spread. The global response infrastructure remains heavily reliant on a single supplier—creating a significant challenge for health systems facing potential scale-up needs. In contrast, GeoVax's GEO-MVA program is being developed as a U.S.-based alternative that uses a next-generation manufacturing platform to increase yield, reduce production time, and eliminate reliance on complex legacy materials. Key updates: - Clinical-grade material for GEO-MVA has been completed, with final packaging expected by Q4 2025.
- Trials are targeted to begin in 2026.
- GeoVax's process is built for scalability and rapid domestic deployment.
The company reported that its GEO‑MVA platform was included in the U.S. government's Rapid Response Partnership Vehicle (RRPV) portfolio, managed by the Biomedical Advanced Research and Development Authority (BARDA). This initiative supports the development of rapid-response medical countermeasure platforms aimed at improving domestic preparedness. According to (GOVX), its inclusion in the RRPV is intended to help advance scalability, U.S.-based manufacturing systems for potential outbreak response. 
7 Reasons Why (GOVX) Will Be Topping Our Watchlist Tomorrow Morning —Wednesday, July 30, 2025
1. Limited Float: (GOVX) has fewer than 14M shares listed in the float—which could set the stage for significant swing potential if demand begins to shift. 2. Analyst Coverage: Noble Capital Markets recently maintained an Outperform rating and issued a $10 target for (GOVX), which suggests over 1,100% upside potential from today's (Tuesday, 7/29) open. 3. GeoVax Advances Gedeptin Combo Trial: GeoVax is launching a Phase 2 trial combining Gedeptin® with pembrolizumab to improve event-free survival in resectable head and neck cancer, building on KEYNOTE-689's success. 4. Federal Signal Boost: (GOVX) confirmed its platform was included in BARDA's Rapid Response Partnership Vehicle (RRPV), supporting advanced U.S.-based bioproduction platforms. 5. Multi-Program Pipeline: (GOVX) is currently advancing initiatives in immune defense, oncology, and domestic countermeasure systems—all showing active progression in 2025. 6. Manufacturing Advantage: The company's proprietary cell-line technology has already produced clinical-grade material, with packaging expected in Q4 and trials set for 2026. 7. Market-Backed Strategy: Crystal Research Associates cited (GOVX)'s programs as targeting market segments projected to exceed $30B, $15B, and $10B, respectively. Start Your Own Research On (GOVX) Before Tomorrow Morning…
GeoVax Labs (Nasdaq: GOVX) continues to stand out as one of the more tightly held and strategically positioned companies we've reviewed this quarter. With fewer than 14M shares in the float, confirmed progress across multiple programs, and active interest from both analysts and federal response teams, this platform is aligning with several fast-moving global themes. The combination of its recent momentum, next-gen manufacturing, and substantial market focus reinforces why (GOVX) is showing up at the top of our screens. We will have all eyes on (GOVX) tomorrow morning—Wednesday, July 30, 2025. Start your own research on (GOVX) before you call it a night. Also, keep a look out for my morning update—it could be hitting bright & early. Have a good night. Sincerely,
Alex Ramsay
Co-Founder / Managing Editor Krypton Street Newsletter | 




No comments:
Post a Comment