*Sponsored
Krypton Street Announces Coverage On Autonomix Medical, Inc. (NASDAQ: AMIX) Starting This Morning—Monday, July 21, 2025
(AMIX) Comes Backed By Several Potential Catalysts Including:
Recent Movement Has Lifted (AMIX) Above Its 5-Day, 20-Day, And
50-Day Moving Averages.
Analysts From Maxim And Ladenburg Have Recently Issued $5 And $18 Targets On (AMIX), Which Suggest 230% And 1,100% Potential Upsides.
Last Time This Hit Our Radar, (AMIX) Opened At $1.37 And Tapped $2.43 Within Hours—Marking An Approximate 77% Intraday Move.
With Its Float Under 2.2M Shares, (AMIX) Could Witness the Potential For Big Swings If Demand Begins To Shift.
Pull Up (AMIX) This Morning—While It's Still Quiet…
July 21, 2025 At The Bell | Why (AMIX) Just Took the Top Spot on Our Radar Dear Reader, Here we are, the bell just rang, and (AMIX) is headed straight to the top of our watchlist. Some setups demand more than a second glance—and this morning, one name is rising above the noise. Autonomix Medical, Inc. (NASDAQ: AMIX) is back in focus with a rare combination of momentum: fast-moving technicals, newly issued analyst targets, and key progress in clinical development. It's not the first time we've seen this one make a move—and if early signs hold, it won't be the last. The last time we brought you (AMIX)—just a few weeks ago on June 27—it opened at $1.37 and tapped $2.43 within a few hours, marking an approximate 77% intraday move. Today, we're watching closely once again, as new potential catalysts begin to emerge. Based on what we're seeing, (AMIX) is quickly becoming one of the most compelling setups on our radar this morning—Monday, July 21, 2025.
According to MarketWatch, (AMIX) has fewer than 2.2M shares in its public float—placing it among the tightest floats currently listed on Nasdaq. When a company has a float this small, it doesn't take much for it to see big swing potential, when demand begins to shift. Just this month, two institutional analysts released coverage with head-turning upside targets: - Last week, Maxim Group's Anthony Vendetti reiterated coverage on (AMIX) with a $5 target, which suggests over 230% upside potential from its recent $1.50.
- Then, this past Friday, July 18, 2025, Jeffrey Cohen of Ladenburg Thalmann raised the bar dramatically—publishing a note assigning an $18 target on (AMIX), which from its recent range, suggests over 1,100% upside potential.
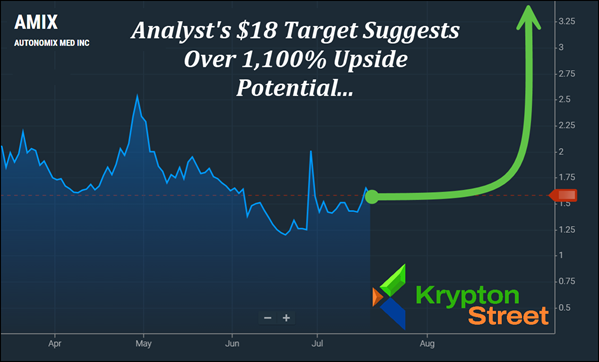
Right now, (AMIX) is trending above several critical technical markers—including its 5-Day, 20-Day, and 50-Day moving averages—pointing to potential momentum building beneath the surface. With the 100-Day and 200-Day averages at $1.80 and $4.16, respectively, (AMIX) may now be approaching longer-term breakout zones that could draw serious attention. The technical setup is one we're watching closely. Keep reading to see why (AMIX) is topping our watchlist this morning—Monday, July 21, 2025.
Autonomix Medical, Inc. (NASDAQ: AMIX): Precision-Guided
Innovation in Cancer Pain Care
See Company Presentation Deck Here (AMIX) is working to transform how pain is addressed in some of the most difficult medical cases—starting with advanced cancers like pancreatic. Backed by strong early clinical results, a growing patent portfolio, and a leadership team with a proven track record, Autonomix is developing a new kind of treatment system that goes far beyond today's standard methods. At the center of their platform is a specialized device powered by a proprietary microchip—built to detect and interpret nerve activity with remarkable accuracy. 
This chip is small enough to get close to the source of pain and smart enough to guide real-time treatment, allowing doctors to pinpoint overactive nerves, treat them directly, and confirm success in one seamless procedure. Unlike current techniques that rely on indirect targeting or blind ablation, Autonomix's system gives physicians real-time feedback—offering greater confidence and control during treatment. This targeted approach may help reduce complications, lower opioid use, and open doors to broader nerve-related care. Tackling a Critical Pain Challenge
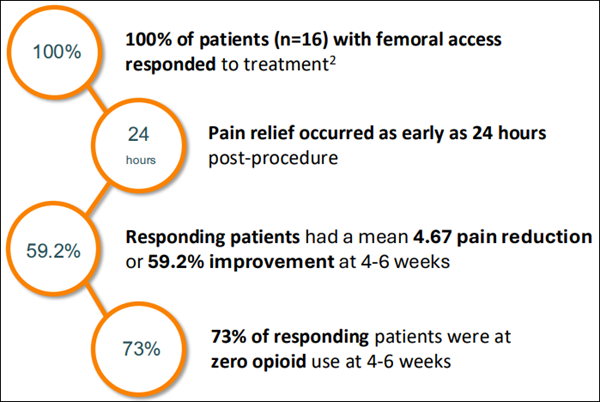
Pancreatic cancer is one of the most aggressive forms of cancer, and for many patients, pain becomes overwhelming and difficult to manage. In Autonomix's first-in-human clinical trial, the company treated 20 patients facing this exact issue—and the results were significant. - 100% of patients treated through one specific access point responded to the procedure
- Pain relief began in as little as 24 hours
- After 4–6 weeks, 73% of responders no longer needed opioids
- No serious complications related to the device or the procedure were reported
These outcomes point to more than just symptom relief—they highlight the potential for a safer, more targeted way to manage pain when few other options exist. A Smarter, More Targeted Platform
What sets Autonomix apart is how their system works. It doesn't rely on traditional approaches that often treat the entire area or require broad interventions. Instead, the company's platform uses a combination of real-time feedback and precision tools to treat only the nerves causing the issue—while confirming during the procedure that the treatment is working. This is a smarter, more focused method of pain management that may also be applied to other serious conditions involving nerve activity. What's Next: Clinical Expansion Underway

Following the success of its first trial, Autonomix is launching a second phase—expanding its platform to include a broader range of cancers that also cause intense nerve-driven pain. These include earlier-stage pancreatic cancer, as well as liver, bile duct, and gall bladder cancers. This expanded study is expected to begin in Q2 2025 and could significantly increase the clinical scope of Autonomix's system. The company remains on track for a 2026 FDA submission, with a long-term path toward broader regulatory clearance. With breakthrough technology, early clinical traction, and a clear expansion plan, Autonomix is aiming to redefine how cancer pain is managed—and possibly much more. Follow-On Clinical Phase Now Underway
Autonomix recently announced the enrollment and treatment of the first patient in its follow-on human clinical study, known as PoC 2—marking a major milestone in its multi-indication growth strategy. This new phase builds directly on the success of its PoC 1 trial and doubles the potential addressable market beyond pancreatic cancer by expanding into earlier-stage pancreatic, liver, bile duct, and gall bladder cancers—all tied to nerve-driven pain through the celiac plexus. According to CEO Brad Hauser, this milestone reinforces Autonomix's momentum and further validates the platform's potential to address a broader range of high-need visceral cancer indications. Longer term, the company is also exploring additional clinical directions in cardiology, hypertension, and chronic pain, highlighting the broader platform opportunity still to come. A Platform Built by Veterans
Autonomix isn't just betting on technology—it's backed by a leadership team with a track record of execution. CEO Brad Hauser previously led exits totaling over $3B, including a $550M acquisition by AbbVie and a $2.4B sale to Allergan. Other key executives and co-founders also bring deep medtech experience and have led companies through commercialization, regulatory approval, and large-scale acquisitions. With more than 120 issued and pending patents, Autonomix is working to secure a durable IP moat around its proprietary systems and signal-processing technologies. Recent Developments
Over the past several weeks, (AMIX) has released a series of updates that underscore both the momentum behind its clinical program and the growing institutional confidence in its proprietary platform. July 2025: PoC 2 Clinical Phase Begins
Autonomix announced the treatment of the first patient in its follow-on PoC 2 study, aimed at expanding clinical validation into additional high-need visceral cancers beyond pancreatic. The PoC 2 phase builds on the success of PoC 1 and is expected to double the company's potential clinical reach, marking a key inflection point in both strategy and addressable market scope. June 2025: Clinical Expansion Approved
Autonomix received Ethics Committee authorization to begin PoC 2, the next phase of its clinical program. This study expands beyond pancreatic cancer to include liver, bile duct, and gall bladder cancers—conditions tied to intense nerve-driven pain. With enrollment expected to begin soon, (AMIX) is now targeting a broader clinical field that could double its reach. May 2025: Key U.S. Patent Granted
In a major milestone, Autonomix was granted U.S. Patent No. 12,257,071, covering its real-time nerve-mapping and ablation technology. The patent protects innovations like embedded sensors, adaptive controls, and full-system integration—further strengthening (AMIX)'s edge in treating nerve-driven conditions with precision and minimal invasiveness. April–May 2025: Breakthrough Clinical Results
Autonomix shared strong results from its first human trial treating pancreatic cancer pain. 100% of targeted patients responded, with pain relief starting in 24 hours, and 73% off opioids by week six. These results confirmed both the safety and power of the company's "Sense, Treat, Verify" approach—and set the stage for its upcoming expansion into other high-need areas. Positioned for the Future
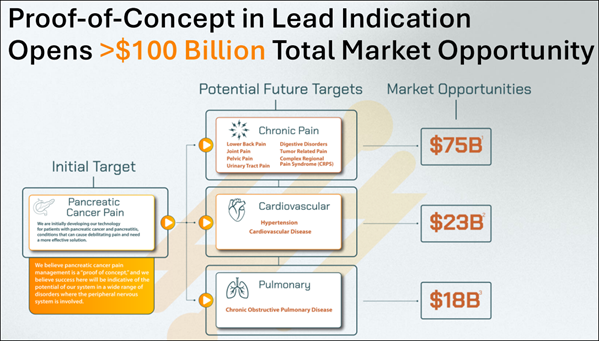
Autonomix is targeting a total combined market potential exceeding $100B, with focus areas spanning pain management, neurostimulation, and visceral nerve-related indications. Its minimally invasive system stands out in a field crowded by blind ablation procedures and signal-blind diagnostics. As new trials begin and technical progress continues, (AMIX) remains a name worth watching in the emerging class of neural-targeted precision therapies. 7 Reasons Why (AMIX) Is Topping Our Watchlist This Morning
—Monday, July 21, 2025….
1. Technical Crossovers Building: Recent momentum has lifted (AMIX) above its 5-Day, 20-Day, and 50-Day moving averages—and the last time we highlighted it, on June 27, it moved approximately 77% within hours.
2. Very Limited Float: With fewer than 2.2M shares available in the public float, (AMIX) remains one of the most compact issuers currently listed on Nasdaq. 3. Analyst Eyes Watching: Two institutional firms—Maxim Group and Ladenburg Thalmann—have issued targets of $5 and $18, reflecting approximately 230% and 1,100% upside potential from recent levels. 4. Early Trial Results: Initial human data showed a 100% response rate from one targeted access point, with 73% of responders eliminating opioid use within weeks. 5. Platform Expansion Underway: (AMIX) recently began treatment in its PoC 2 clinical phase—doubling its target scope to include liver, gall bladder, and bile duct cancers. 6. Leadership Track Record: The executive team behind (AMIX) includes names tied to multiple large-scale exits—including a $2.4B deal with Allergan and a $550M acquisition by AbbVie. 7. Patent Edge Growing: With a new U.S. patent granted in May 2025, (AMIX) now holds more than 120 issued and pending patents covering its core system and chip-based targeting technology. Pull Up (AMIX) This Morning While It's Still Quiet…
With a float under 2.2M shares, a wave of technical signals flashing, and two institutional targets suggesting outsized upside potential, Autonomix Medical, Inc. (NASDAQ: AMIX) has just landed on our radar in a big way. Early human trial data, a next-phase clinical expansion already underway, and a deep IP portfolio backed by proven leadership only add to the momentum building behind this name. Remember, the last time we brought you (AMIX)—just a few weeks ago on June 27—it opened at $1.37 and tapped $2.43 within a few hours, marking an approximate 77% intraday move. We already have (AMIX) at the very top of our screen this morning. Pull this up while it's still quiet. The bell just rang, and (AMIX) is headed straight to the top of our watchlist. And get ready—my next update could be out any moment. Sincerely, Alex Ramsay
Co-Founder / Managing Editor Krypton Street Newsletter
| 






No comments:
Post a Comment